About
Humans and viruses live in shared environments where changes in behavior or genetics impact each other. Urban areas, as hubs of activity where people interact frequently, represent ideal places to monitor human-virus interactions. The Human Papillomavirus (HPV) has many genotypes, some of which cause cancer that are vaccine-preventable. With HPV vaccines now broadly available in Austria since 2023, a system is needed to assess vaccine uptake and changes in HPV genotypes.
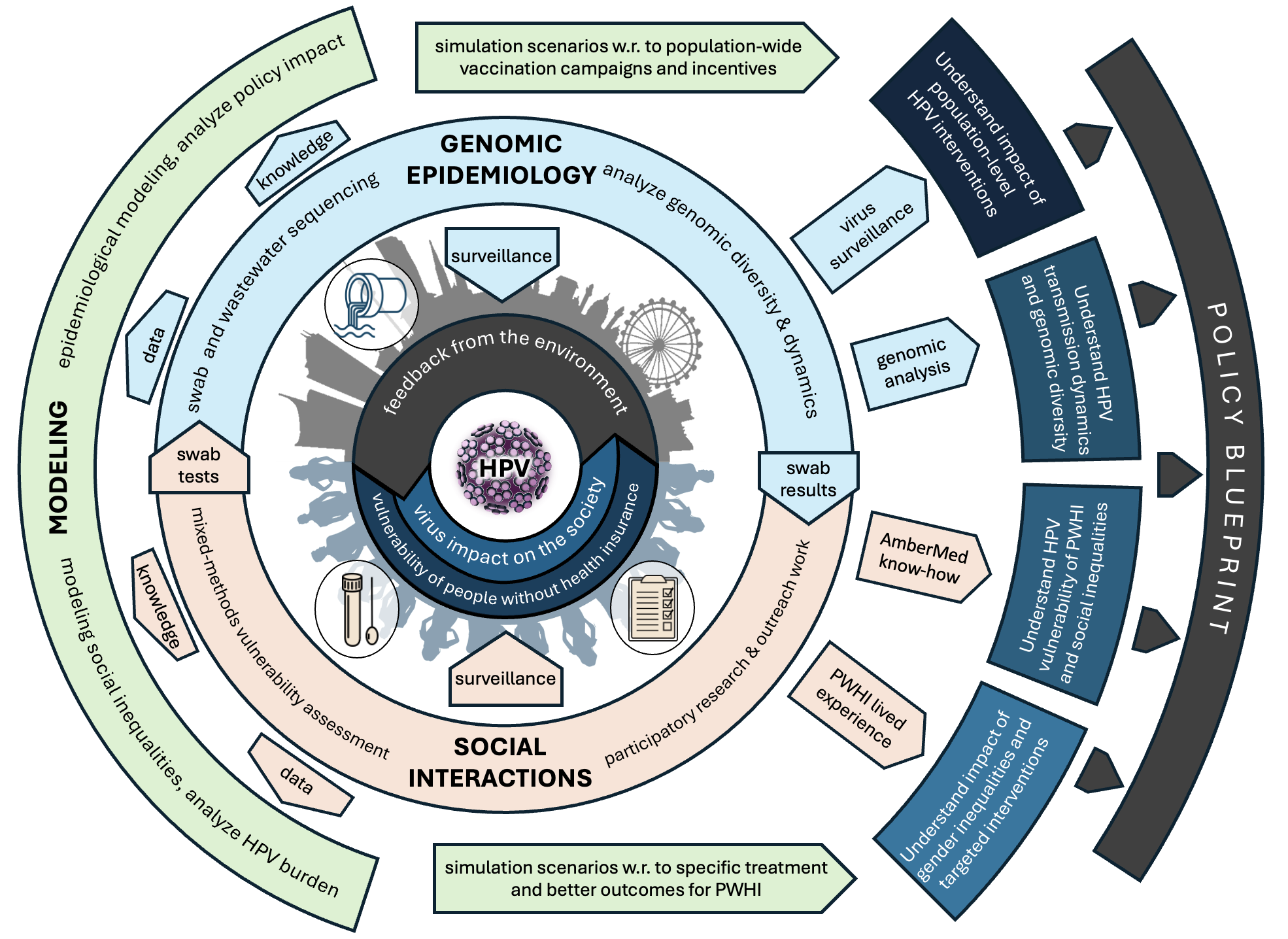
For this, HPVienna assembled a working partnership of four complementary disciplines: virology to examine the virus, social science and public health to understand human behavior, and mathematics to build dynamic systems models able to describe interactions and detect changes over time. A particular focus will lie on a highly vulnerable population: people without health insurance. Our ultimate aim is to better understand the spread of HPV everywhere and reduce the burden of disease.
Scientific summary
In 2023, Austria extended its national vaccination program for Human Papillomavirus (HPV), joining WHO’s call to eliminate cervical cancer and expanding its goals with a gender-neutral policy. Yet, the lack of any HPV surveillance system poses severe challenges in assessing the impact of vaccination, the evolutionary consequences on circulating HPV genotypes, and the basic sociality of HPV within dense urban environments. This project proposes a novel transdisciplinary approach which integrates sequencing-based genomic epidemiology, mixed-method social science and public health research, and agent-based modeling to tackle these interconnected challenges. We will map viral population dynamics onto urban social dynamics, unveiling transmission networks and contributing to prevention of HPV-linked cancers. Partnering with the NGO and clinic Ambermed that supports patients without health insurance in Austria, volunteer HPV swabbing will be enhanced through social participatory methods, and complemented by a novel pipeline to detect population-wide circulating HPV genotypes from wastewater to test the risk of high-impact variants and indirect effects of vaccination. All novel and complementary evidence will be synthesized to yield a transmission model for the whole Viennese population, intervention scenarios, and a policy blueprint with recommendations and tailored measures.
Our pioneering approach tackles existing socioeconomic inequalities and gender disparities with an additional focus on boys and men. This transdisciplinary strategy pushes the boundaries of OneHealth in practice, with the potential of serving as a prototype for effective prevention and multi-system surveillance of infectious diseases in the urban environment more generally.